Past Issues
Acute Coronary Syndrome (ACS) in Polycythemia Vera: A Case Report with Review of Literature
Abhishek Shah1, Charan Reddy KV1*, Abhay Bhave2, Prakash Sanzgiri1
1 Department of Cardiology, Lilavati Hospital and Research Center, Bandra (W), Mumbai-50, India 2 Department of Hematology, Lilavati Hospital and Research Center, Bandra (W), Mumbai-50, India
*Corresponding Author: Charan Reddy KV, Department of Cardiology, Lilavati Hospital and Research Center, Bandra, Mumbai-50 India, E-mail: [email protected]
Received: September 18, 2019 Published: October 23, 2019
ABSTRACT
Polycythemia Vera (PV) is a chronic bone marrow disorder which causes hyper viscosity of blood thereby, increasing the risk of coronary thrombosis and acute myocardial infarction (MI). We report a 65-year-old male patient with history of PV presented with ST segment elevation hyper-acute anterior wall myocardial infarction (MI). His blood investigation report showed higher levels of hemoglobin (Hb) and hematocrit (Hct). This case illustrates the importance of recognizing PV as a major cause of thrombotic occlusion of coronary arteries and to prevent future ischaemic events by initiating suitable therapy.
Keywords: Myocardial infarction, Polycythemia Vera (PV), JAK2V617F mutation
INTRODUCTION
Myocardial infarction (MI) and heart failure (HF) are the most common cause of death in the world [1]. Traditional risk factors for MI are hypertension, diabetes, dyslipidemia, smoking and a family history of premature coronary heart disease (CHD) [1]. Polycythemia Vera (PV) is a rare type of blood cancer in which body produces too many red blood cells. If not treated, PV can lead to life-threatening complications [2-4]. This disorder is marked by increased bleeding and thrombotic occlusion of coronary arteries leading to morbidity and mortality in 40% to 60% of the patients [3].
Patients with a prior history of vascular thrombosis and who are in advanced age (≥ 60 years) are at a higher risk for complications [3,5]. The rate of incidence of PV is higher among men than women (~2.8 per 100,000 men and ~1.3 per 100,000 women) [6]. The overall incidence is 2.30/1,00,000 persons/year [7]. Cytoreductive treatment by phlebotomy or chemotherapy and antiplatelet therapy with low-dose aspirin have significantly reduced the number of thrombotic complications and substantially improved survival [8].
CASE PRESENTATION
A 65 year old non-diabetic and non-hypertensive male presented to our hospital with severe retrosternal compressive chest pain radiating to left arm with sweating since the past one hour. On physical examination, his pulse rate and blood pressure (BP) was recorded as 96/min and 110/80 mmHg respectively. Patient is a non-smoker and without any history of drug use or a family history of coronary artery disease (CAD). On auscultation, there was no S3 gallop or any cardiac murmurs, but had bilateral basal crackles (rales) in the lung fields. The oxygen saturation was 92% on room air. However, patient was a known case of Polycythemia Vera (PV) (positive for JAK2 V617F mutation) diagnosed 2 years back. He was on hydroxyurea for one year, which was stopped due to recurrent infections and altered platelet counts. The patient underwent Phlebotomy six months back, there after he was asymptomatic.
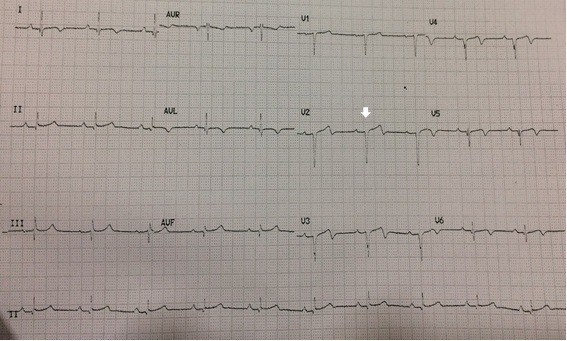
Electrocardiogram (ECG) showed hyperacute anterior wall myocardial infarction (Figure 1). Patient was given loading doses (LD) of dispersible Aspirin (325 mg), Ticagrelor (180 mg), Atorvastatin (80 mg) and intravenous Furosemide (40 mg). 2-Dimensional echocardiography (2D-ECHO) showed regional wall motion abnormality with hypokinesis of the entire anterior wall with preserved wall thickness. The left ventricular ejection fraction (LVEF) was 35%. He was immediately shifted to cardiac catheterization lab for primary angioplasty.
Figure 1: 12-lead Electrocardiogram (ECG) obtained at the admission showing hyper acute anterior wall myocardial infarction (>2 mm ST segment elevation in V2-V4 with reciprocal changes in inferior leads, III and avF). (Arrow indicates ST-segment elevation in the anterior leads).
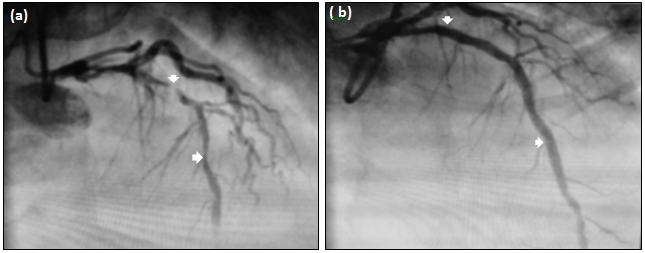
Coronary angiogram (CAG) was performed through the right radial artery route, which revealed normal left main and it’s bifurcation, but with two lesions of critical 99% thrombotic subtotal occlusion in the proximal LAD and a 80% stenosis in mid LAD with thrombolysis in myocardial infarction-II flow (TIMI-II) (Figure 2a). Left Circumflex (Lcx) was non-dominant and did not show any significant lesion. Right coronary artery (RCA) was normal.
Figure 2a & 2b: (a) Coronary angiography (CAG) revealed normal left main with two significant lesions, one of 99% thrombotic subtotal occlusion in proximal left anterior descending (LAD) and other 80% stenosis in mid LAD with thrombolysis in myocardial infarction-II (TIMI-II) flow. (b) Percutaneous Transluminal Coronary angioplasty (PTCA) to LAD with two overlapping Drug Eluting Stents (DES). (Arrows indicate lesion status before PTCA (a), after PTCA (b).
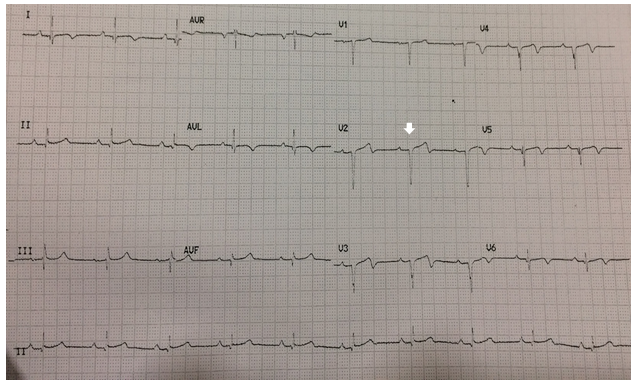
Percutaneous Transluminal Coronary Angioplasty (PTCA) to LAD with two Xience Alpine Everolimus Drug Eluting Stents (DES) overlapping each other was performed. The thrombus burden was reduced with the use of thrombus aspiration catheter (6F EXPORT catheter, Medtronic, USA) and intravenous Abciximab (a glycoprotein IIb/IIIa receptor antagonist) infusion for 12 hrs (Figure 2b). Total Door to Balloon Time (DBT) was about 40 minutes. Post procedure, there was ST segment resolution (more than 50% within 60 minutes on serial ECG’s) with significant reduction in symptoms of chest pain (Figure 3). He was shifted to Cardiac Intensive Care Unit (C-ICU) in a stable condition and was discharged on day three after taking the opinion of a Hematologist. Patient was discharged on dual antiplatelet drugs, ticagrelor (90 mg twice a day) aspirin (75 mg/day) and atorvastatin (40 mg/day). Hydroxyurea (15 mg/kg/day) was restarted by the hematologist. He is currently on a regular follow-up and is doing well.
Figure 3: ECG taken Post procedure (after 1 hr) showing ST segment resolution (more than 50%) in the anterior leads,V2 to V4 (Arrow indicates ST segment resolution in the anterior leads).
DISCUSSION
Polycythemia Vera (PV) is a chronic myeloproliferative neoplasm, involving a multipotent hematopoietic progenitor cell lines resulting increased production of RBCs, granulocytes and platelets. Erythrocytosis with increased hematocrit (high blood viscosity) and platelet count generate microparticles (0.1 μm to 1 μm) [5,9] with platelet dysfunction and leucocyte-platelet conglomerates that leads to thrombus formation [5,9,10].
MI can occur in PV patients in the absence of any cardiac risk factors [6]. Our patient reports revealed higher levels of hemoglobin (Hb) (20.65 g/dl), packed cell volume (PCV) 60%, WBC (30.43 × 109/L) and platelet (343 × 109/L) counts [11,12]. We also determined the levels of lipids such as total cholesterol and LDL and found 146 mg/dl of 88 mg/dl respectively. Since, there is reduced life expectancy in PV patients, and also risk of transformation to myelofibrosis and acute myeloid leukemia, consultation with haematologist was necessary.
Early diagnosis and treatment of MI in PV is challenging and should be carefully managed using a multi-disciplinary approach. In this respect, meager information is available on the treatment of MI in PV patient’s hence we can’t expect answers to all the queries. For instance: 1) What could be the best option for revascularization in PV patients? 2) Which one is first, cytoreduction or revascularization or both together? 3) Which anti-platelet drug is preferred and at what doses? 4) Heparin or Bivalirudin and need for glycoprotein (GP)IIb/IIa receptor antagonists?, 5) Whether long-term treatment with Non-vitamin K antagonist oral anticoagulants (NOACs) or Vit K antagonists is required post-PTCA? and 6) Whether cytoreductive agents can be given long term or repeated phlebotomy is better?. Although, antiplatelet and cytoreductive drugs (e.g. phlebotomy, hydroxyurea or both) are prescribed [13], but it is uncertain which type of coronary reperfusion procedure is appropriate for treating MI in PV patients. The safety and efficacy of fibrinolytic therapy as the treatment of choice for acute STEMI in patients with PV is yet to be established. Effective fibrinolytic therapy may still be associated with residual coronary thrombus, which is more likely seen in PV patients who have high platelet counts [14].
Cytoreductive treatment with phlebotomy to achieve hematocrit of ≤45% is critical in optimizing the outcomes in patients with PV [5]. Phlebotomy and myelosuppression are the treatment options most often utilized, either alone or in combination. However, therapeutic phlebotomy to reduce hematocrit levels before primary angioplasty can lead to unnecessary delay and should not be pursued in an acute setting. Hyper-viscosity and platelet aggregation can increase the risk of stent thrombosis [15], making the patient vulnerable, due to post procedure drop in the levels of anticoagulation. Therefore, in our patient, it was decided to perform phlebotomy after the PTCA, to maintain the haematocrit level below 45%, and therapy with dual anti-platelet medication and Hydroxyurea. Long term hydroxyurea can also reduce the risk of PV transformation to post fibrotic myelofibrosis and acute myeloid leukemia.
Patients after a thrombotic episode with PV would be on both Aspirin and Hydroxyurea. The benefit of hydroxyurea is not only as a cytoreductive but has shown reduce risk of repeat thrombosis when given with aspirin alone. Willoughby, et al. [7] reported that prophylactic aspirin use in patients with PV reduced the occurrence of major vascular events by 22% and nonfatal myocardial infarction by 30%. However, it has also shown that MI patients with PV exhibit recurrent stent thrombosis, despite appropriate anti-platelet therapy due to underlying hyper-viscosity [15]. This study further revealed addition of GPIIb/IIIa receptor blockers like Abciximab reduced the risk of acute and sub-acute thrombosis, but led to increased risk of bleeding. Hence, this treatment should be considered on case to case basis to reduce the risk of adverse events. Friedrich, et al. [16] have reported a rare case of splenic rupture following the use of GPIIb/IIIa antagonist for MI patients with PV. It has becomes imperative to use best possible anti-platelet agents to prevent post-PTCA stent thrombosis. Long term use of anticoagulation with vitamin-k antagonists or newer oral anticoagulants often lead to increased risk of major bleeding, especially in elderly population. Hence, current evidence supports using high potency anti-platelet medication (e.g. ticagrelol or prasugrel), aggressive therapeutic phlebotomy and cytoreductive therapy using hydroxyurea [17] or anagrelide [1] post PTCA [18]. Some of the studies dealing the association between ACS and PV are summarized (Table 1).
Table 1: Some of the case reports on the management of AMI and PV in medical literature (Hct: Hematocrit; CABG: coronary artery bypass graft).
|
Age/ Sex
|
Presentation |
Hct (%)
|
Initial Treatment
|
PCI finding |
Intervention |
Post-op treatment
|
Long term treatment
|
Refe rence |
|
68/M |
STEMI
|
65 |
Phlebotomy |
Double vessel disease: 99% stenosis of LAD, 99% stenosis in diatal RCA |
JAK2 mutaion, AMI secondary to PV Mutation |
- |
Not reported |
[19] |
|
66/F |
STEMI
|
57 |
Aspirin, Phlebotomy, Heparin |
Occlusive thrombus in Proximal LAD |
Within 24 hrs Refractory cardiogenic shock developed |
Urokinase- 500,000 units followed by PTCA |
Not reported |
[10] |
|
37/M |
STEMI |
50 |
Aspirin, Plavix, Lovenox |
Not performed due to lack of availability in that facility |
No intervention |
Reteplase 10 units, Bolus 10 units given intravenously
|
Aspirin, Hydroxyurea, Phlebotomy |
[20] |
|
65/M |
Stable angina |
59 |
Aspirin (until day before surgery) Heparin (until morning of surgery), Phlebotomy
|
Proximal LAD showed 99%; Mid LAD showed 75%, OM showed 90% stenoses |
CABG |
Post op day 1 developed STEMI, PCI showed thrombosis of native and graft as well s/p PTCA |
Aspirin, Plavix, Warfari |
[21] |
|
61/F |
Stable angina
|
40 |
Aspirin, Hydroxyurea |
LAD showed 80%, RCA showed 90% stenoses |
CABG |
Heparin in early post operative period |
Aspirin, Plavix, Warfarin, Hydroxyurea |
[22] |
|
34/M |
STEMI |
65 |
Aspirin, Plavix, Heparin, Phlebotomy |
Mid LAD 60% occlusion with a distal thrombotic occlusion |
No intervention |
Phlebotomy |
Not reported |
[23] |
|
42/M |
Unstable angina |
71 |
Aspirin, Heparin, Phlebotomy |
LAD thrombosis distal to perforating branches otherwise normal coronaries |
No intervention |
- |
Warfarin |
[24]
|
|
30/M |
AMI |
61 |
- |
Not performed |
Tissue plasminogen activator 100 mg/2hr |
Developed refractory cardiogenic shock failed resuscitation |
- |
[25] |
|
39/M |
NSTEMI |
53 |
Warfarin |
Venous thrombosis multiple sinuses and internal jugular veins |
Aspirin, Ni- trates, Statin |
CAG showed single vessel coronary artery disease, mid LAD 80% stenosis (Figure 2) distal LAD 100% with thrombus |
|
[26] |
|
73/M |
NSTEMI |
66 |
Aspirin, Heparin, Metoprolol, Simvastatin, Lisinopril |
Not performed |
CABG |
- |
Phlebotomy, aspirin, statin, β-blocker |
[12] |
|
17/M |
STEMI in leads II, III, aVF, ST depression in leads I, aVL, V1-V6 |
36.5 |
Platelet pheresis 3000 mg/day, Hydroxyurea, Antiplatelet agents |
- |
Post splenectomized hemoglobin H-Constant Spring disease, JAK2, Thalassemia with thrombocytosis. |
Thrombolytic therapy or PCI
|
Aspirin, Ticlopidine, Phlebotomy |
[27] |
|
58/M |
STEMI |
|
Aspirin, Clopidogrel |
PCI, coronary stent implanted |
No intervention |
PCI with aspiration thrombectomy Acute stent thrombosis |
Ticagrelor, Hydroxyurea |
[28] |
|
55/M |
STEMI |
65 |
Aspirin, Statin, Heparin Angiotensin-converting enzyme inhibitor |
STEMI in leads V1-V5 with T wave Inversion hypokine sis of the septolateral wall, |
No intervention |
- |
Myelosuppressive therapy with 15 mg/kg per 24 of hydroxyurea |
[3] |
CONCLUSION
Our case illustrates the importance of recognizing PV as an important cause of coronary thrombosis and MI so as to initiate appropriate treatment strategies. It is also important to consider adequate use of myelosuppressive agents such as hydroxyurea or ruxolitinib in high risk patients to prevent future thrombotic events. The search for laboratory markers to detect functional defects in platelets or leukocytes to predict thrombotic events is awaited. New strategies for the management in MI patients with PV undoubtedly have become high priority research in future.
Acknowledgment
The authors are thankful to the patient for consenting us to publish this report. We also thankful to the staff of Clinical and Interventional Cardiology Department, Lilavati Hospital and Research Center for their support during the study.
Conflicts of Interest
None declared.
Source of Funding
None declared
REFERENCES
- Cahill TJ, Kharbanda RK (2017) Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: Mechanisms, incidence and identification of patients at risk. World J Cardiol 9(5): 407-415.
- Turakhia MP, Murphy SA, Pinto TL, Antman EM, Giugliano RP, Cannon CP, et al. (2004) Association of platelet count with residual thrombus in the myocardial infarct-related coronary artery among patients treated with fibrinolytic therapy for ST-segment elevation acute myocardial infarction. Am J Cardiol 94(11): 1406-1410.
- Adel G, Aoulia D, Amina Y, Aymen BA, Abdel-Hamid NM (2013) Polycythemia vera and acute coronary syndromes: pathogenesis, risk factors and treatment. J Hematol Thromb Dis 1: 107-112.
- Malak S, Labopin M, Saint-Martin C, Bellanne-Chantelot C, Najman A, French Group of Familial Myeloproliferative Disorders (2012) Long term follow up of 93 families with myeloproliferative neoplasms: Life expectancy and implications of JAK2V617F in the occurrence of complications. Blood Cells Mol Dis 49(3-4):170-176.
- Pearson TC, Wetherley-Mein G (1978) Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet 2(8102): 1219-1222.
- Polycythemia vera facts. FS13. Leukemia & Lymphoma Society Web site. www.lls.org/content/nationalcontent/resourcecenter/freeeducationmaterials/mpd/pdf/polycythemiavera.pdf 2012. Accessed January 15, 2019.
- Willoughby S, Pearson TC (1998) The use of aspirin in polycythaemia vera and primary thrombocythaemia. Blood Rev 12(1): 12-22.
- Barbui T, Finazzi G (2006) Evidence-based management of polycythemia vera. Best Pract Res Clin Haematol 19(3): 483-493.
- Murphy S, Iland H, Rosenthal D, Laszlo J (1986) Essential thrombocythemia: An interim report from the Polycythemia Vera Study Group. Semin Hematol 23(3): 177-182.
- Venegoni P, Schroth G (1994) Myocardial infarction and polycythemia vera: how should we treat it? Cathet Cardiovasc Diagn 32(3): 259-261.
- Benjamin D, Yeshurun D, Charnilas J, Pinkhas J (1978) Hyperlipidemia and myocardial infarction among 118 patients with polycythemia vera. Am J Med Sci 276(1): 23-26.
- Thota R (2015) Therapeutic challenges in the management of acute myocardial infarction in polycythemia vera. J Blood Disorders Transf 6: 1-3.
- Goethals P, Evrard S, Dubois C (2000) Recurrent coronary stent thrombosis. Acta Cardiol 55(6): 371-373.
- Sazawal S, Bajaj J, Chikkara S, Jain S, Bhargava R, Mahapatra M, et al. (2010) Prevalence of JAK2 V617F mutation in Indian patients with chronic myeloproliferative disorders. Ind J Med Res 132: 423-427.
- Zavalloni D, Marsico F, Milone F, Presbitero P (2004) Is conventional antiplatelet therapy for the prevention of coronary stent thrombosis always safe? A case report of a patient with polycythemia vera. Ital Heart J 5(2): 163-166.
- Friedrich EB, Kindermann M, Link A, Bohm M (2005) Splenic rupture complicating peri-interventional glycoprotein IIb/IIIa antagonist therapy for myocardial infarction in polycythemia vera. Z Kardiol 94(3): 200-204.
- Hosoya H, Hosoya H Levine JJ, Godberg S (2017) Double the trouble: Acute coronary syndrome and ischemic stroke in polycythemia vera. Am J Med 130(6): 237-240.
- Marchetti M, Tartari CJ, Russo L, Panova-Noeva M, Leuzzi A, Rambaldi A, et al. (2014) Phospholipid-dependent procoagulant activity is highly expressed by circulating microparticles in patients with essential thrombocythemia. Am J Hematol 89(1): 68-73.
- Soni P, Patel J, Agarwal N, Rai AK, Kupfer Y (2018) A rare case of polycythemia vera precipitating myocardial infarction. Crit Care Med 46(1): 256-258·
- Bahbahani H, Aljenaee K, Bella A (2015) Polycythemia vera presenting as acute myocardial infarction: An unusual presentation. J Saudi Heart Assoc 27(1): 57-60.
- Osada H, Nakajima H, Meshii K, Ohnaka M (2014) Acute coronary artery bypass graft failure in a patient with polycythemia vera. Asian Cardiovasc Thorac Ann 24(2): 175-177.
- Oz BS, Asgun F, Akay HT, Kaya E, Kuralay E, Tahar H (2007) Anticoagulation after coronary artery surgery in patients with polycythemia vera: report of two cases. J Card Surg 22(5): 420-422.
- Wu CF, Armstrong GP, Henderson RA, Ruygrok PN (2005) Polycythaemia vera presenting as ST-elevation myocardial infarction. Heart Lung Circ 14(1): 51-53.
- Chan AW, Drobac M, Sternberg L (1997) The management of acute myocardial infarction in a patient with polycythemia rubra vera during the thrombolytic era--does it make a difference? Can J Cardiol 13(1): 59-63.
- Hermanns B, Handt S, Kindler J, Fuzesi L (2009) Coronary vasculopathy in polycythemia vera. Pathol Oncol Res 4(1): 37-39.
- Dolai TK, Mandal PK (2004) Non-ST-segment elevation myocardial infarction (NSTEMI) in a 43-year-old man e Uncommon etiology. Ind College Cardiol 4: 277-280.
- Rattarittamrong E, Norasetthada L, Tantiworawit A, Chai-Adisaksopha C, Hantrakool S, et al. (2015) Acute non-atherosclerotic st-segment elevation myocardial infarction in an adolescent with concurrent hemoglobin h-constant spring disease and polycythemia vera. Hematol Rep 7(3): 5941.
- Lee HF, Wang CL, Chan YH (2017) Replacement of clopidogrel with ticagrelor for a patient with polycythemia vera accompanied by repeated myocardial infarction and acute stent thrombosis. J Cardiovasc Med Ther 1(1): 1-4.
Copyright: Shah A, et al. ©2019. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Shah A (2019). Acute Coronary Syndrome (ACS) in Polycythemia Vera: A Case Report with Review of Literature. Cardiac 1(1): 1.
 Abstract
Abstract  PDF
PDF